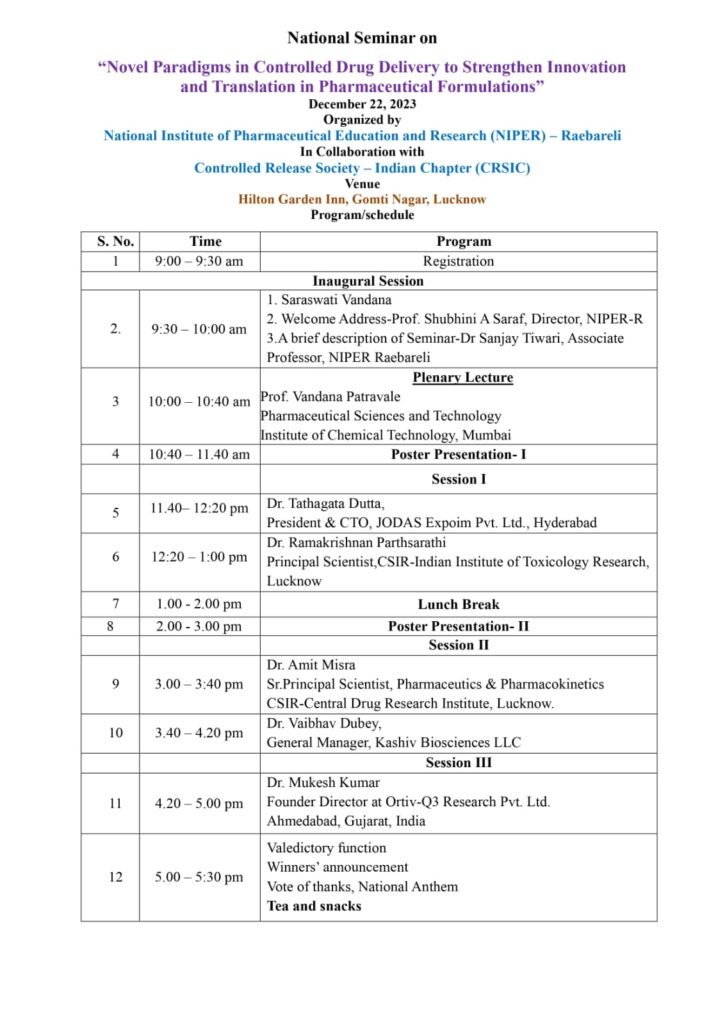
National Seminar – 2023: Novel Paradigms in Controlled Drug Delivery to Strengthen Innovation and Translation in Pharmaceutical Formulations
Local Time
- Timezone: America/New_York
- Date: Dec 21 - 22 2023
- Time: All Day
NIPER Raibareli in association with Controlled Release Society India is organizing National Seminar on 22nd December 2023.
The Seminar showcases eminent speakers and profound researchers
Download the flyer for details.
One-day National Seminar on “LEVERAGING REGULATORY PATHWAY FOR INCREMENTAL INNOVATION WITH SUPERGENERICS”
Local Time
- Timezone: America/New_York
- Date: Aug 03 - 04 2023
- Time: 8:00 pm - 4:00 am
Location
- Smt. Geeta Israni Auditorium
- 6 Floor,Principal K. M. Kundnani College of Pharmacy, Cuff Parade, Mumbai
PRINCIPAL K. M. KUNDNANI COLLEGE OF PHARMACY
In Collaboration with
Controlled Release Society (CRS) Indian Local Chapter
Organises
One-day National Seminar on
“LEVERAGING REGULATORY PATHWAY FOR INCREMENTAL INNOVATION WITH SUPERGENERICS”
FRIDAY, 4 AUGUST, 2023
9:00AM – 5:00PM
VENUE: Smt. Geeta Israni Auditorium, 6 Floor,Principal K. M. Kundnani College of Pharmacy, Cuffe Parade, Mumbai 400 005
All participants have to register for the seminar. Registration fees is Rs. 590/- (inc GST)
Registration link:
https://forms.gle/w9XpRFj7gBqqN4ry8
To download brochure click below:
Novel Paradigms in Drug Delivery
Local Time
- Timezone: America/New_York
- Date: Apr 13 2023
- Time: 2:00 am - 4:15 am
Location
- Online Webinar
Speakers
-
 Harshad Shete
Harshad SheteBusiness Development Manager
(Avanti Polar Lipids-Adjuvants Portfolio)
Croda India Company Private Limited,
Navi Mumbai -
 Indupal Kaur
Indupal KaurProfessor and Chairperson
University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh
US-Fulbright Fellow 2017-18 -
 Varsha Pokharkar
Varsha PokharkarDean, Faculty of Pharmaceutical Sciences &
Vice Principal, Poona College of Pharmacy
Bharati Vidyapeeth (Deemed University),
Pune
Computer Aided Drug Delivery
One Day National Seminar on advances in Cancer Diagnostics and Therapeutics
Local Time
- Timezone: America/New_York
- Date: Jan 05 2022
- Time: 2:00 am
Speakers
-
 Dr. Krutika K SawantDean, Faculty of Pharmacy, Maharaja Sayajirao University, Baroda
Dr. Krutika K SawantDean, Faculty of Pharmacy, Maharaja Sayajirao University, BarodaProf. Krutika K Sawant is the Head, Pharmacy Department, (since 2-5-2017) and Dean, Faculty of Pharmacy (since Oct 2019), The Maharaja Sayajirao University of Baroda, Vadodara.
She obtained her B Pharm, M Pharm and Ph D from The M S University of Baroda.
Her Broad area of Specialization is Formulation and Development of Controlled and Novel Drug Delivery Systems.
She has more than 33 years of Teaching and Research Experience.
She has 1 Indian patent granted, 5 Book chapters, 110 Publications and nearly 80 Papers Presentations.
Prof Sawant has 4548 Citations with h-index of 36 and i10-index of 73.
She has guided 25 Ph D and 82 M Pharm students.
She is a Reviewer for more than 25 Scopus Indexed peer Reviewed International Journals. She is a Member of Board of Studies in Pharmacy and Faculty Board of MSUB and few other Universities.
She has acted as a Resource person for various Seminars, Workshops, Conferences and Staff Development programs.
She is a Member, Research Monitoring Committee / Doctoral Advisory Committee for Gujarat Technological University, Nirma University, CHARUSAT University, Dharamsinh Desai University, Parul University etc.
She has also acted as Examiner for B.Pharm., M.Pharm. and Ph.D. courses at various Colleges and Universities. -
 Dr. Swati BiswasAssociate Professor, Dept. of Pharmacy, BITS Pilani, Hyderabad
Dr. Swati BiswasAssociate Professor, Dept. of Pharmacy, BITS Pilani, HyderabadProfessor Swati Biswas completed B. Pharm and M. Pharm from Jadavpur University, Kolkata, and earned a Ph.D. degree in 2008 from the Department of Pharmaceutical Sciences, Wayne State University, Detroit, Michigan. Later, she worked as a postdoctoral fellow at Wayne State University for one year and Northeastern University for four years. Professor Biswas joined BITS-Pilani Hyderabad as an Assistant Professor in 2013 and was later promoted to Associate Professor in 2018. Her current research interest includes drug delivery to cancer and the eye via nanoparticles systems such as liposomes, polymeric micelles, solid lipid nanoparticles, and inorganic nanoparticles. The lab is actively working on developing nanomedicines capable of executing multiple functions, such as combination drug delivery, chemo-photodynamic therapy, photothermal-chemotherapy, etc., for successful tumor regression. Prof. Biswas has 80 peer-reviewed publications in reputed journals, four book chapters, sixty conference proceedings, and ten patents (Indian and international) to her credit. Prof. Biswas received research supports from DST-Fast Track, DST-SERB, DBT-BioCARe, DST-Indo-Belarus, CSIR, ICMR-DHR, Daewoong Pharmaceuticals, Ltd, Hyderabad, Dr. Reddy’s Laboratory, Hyderabad, and Sun Pharmaceutical Advanced Research Company, Vadodara. Dr. Biswas has been featured among the world’s top 2 % scientists in the single year 2019 and 2020 in a publication of science-wise author databases by Stanford University. Dr. Biswas has recently been nominated as an Editorial Advisory Board Member of the Journal, ACS Molecular pharmaceutics for 2021-2023.
One Day National Seminar on Advances in Cancer Diagnostics and Therapeutics
Organized by SWVSM’s Tatyasaheb Kore College of pharmacy, Warananagar.
Date: 5th January, 2022; 4pm Onwards
Zoom Link: https://bit.ly/3eEarGh
or
Watch on YouTube: https://youtu.be/tNH_FXQM30k
Click here to download flyer: CRSIC-TKCP National Seminar New
One Day National Webinar on “Nanotechnology in Healthcare: Opportunities and Challenges”
Local Time
- Timezone: America/New_York
- Date: Oct 27 - 28 2021
- Time: 7:00 pm - 5:00 am
ORGANIZED BY:
Department of Pharmaceutics
Institute of Pharmacy, Nirma University S.G. Highway, Ahmedabad – 382481, Gujarat, India Website: https://pharmacy.nirmauni.ac.in/
E-mail: CRSIC.Nirma@nirmauni.ac.in
In association with
CONTROLLED RELEASE SOCIETY INDIAN CHAPTER
Brochure: CRS Webnair
Nanotechnology in Healthcare: Opportunities and Challenges
Department of Pharmaceutics, Institute of Pharmacy, Nirma University
in association with
Controlled Release Society Indian Chapter
organizes
One Day National Webinar on
Nanotechnology in Healthcare:
Opportunities and Challenges
28th October, 2021 (Thursday)
Announcement Flyer
CRS-Webinar-Scientific-Schedule-Announcement
Event Brochure
Manufacturing, Characterization and Applications of Monoclonal Antibodies
Local Time
- Timezone: America/New_York
- Date: Oct 10 2020
- Time: 5:30 am - 8:30 am
More Info
Speakers
-
 Dr. K. RajeshwariFounder and Managing Director, Bioklone Biotech Private Limited, Chennai, India
Dr. K. RajeshwariFounder and Managing Director, Bioklone Biotech Private Limited, Chennai, IndiaDr. K. Rajeshwari, Founder and Managing Director of Bioklone Biotech Private Limited, Chennai, India is a scientist turned entrepreneur. She obtained her Ph.D from Indian Institute of Science (IISc), Bangalore, India. She was a Post-Doctoral Fellow at Thomas Jefferson University, Philadelphia, USA and a Visiting Fellow at Tata Institute of Fundamental Research (TIFR), Mumbai, India before inception of Bioklone in 2006. Rajeshwari has been a resource person for several Entrepreneurship development programmes and has been a mentor for aspiring and start-up entrepreneurs. In 2015, Entrepreneurship Development Institute of India (EDII), Gandhi Nagar, Gujarat, India under the Aegis of Department of
Science & Technology published Dr. Rajeshwari’s case study in the science and technology space of a book entitled “The Innovators-Stories of Hi-tech Entrepreneurs”. -
 Dr. Sachin DubeyDeputy Director & Head of the Formulation Analytical Development, Ichnos Sciences, Switzerland
Dr. Sachin DubeyDeputy Director & Head of the Formulation Analytical Development, Ichnos Sciences, SwitzerlandDr. SachinDubey is presently working with Ichnos Sciences, Switzerland as Deputy Director & Head of the Formulation, Analytical & Drug Product Development. His current responsibilities include designing & executing product development & characterization strategies for both early & late-stage products for Ichnos Biologics. Sachin earned his Ph.D. from the University of Geneva, Switzerland & has previously worked with Glenmark Pharmaceuticals, Switzerland &NovozymesBiopharma, Denmark. He has ~ 12 years of experience in biopharmaceutical formulation & combinational product development. Twelve products developed by him & his team are currently in different clinical trials, he has overseen the production of >75 drug product/diluent/diluent for clinical trial phases 1-3. He has extensive experience with regulatory submissions in the USA, EU & India and have 9 PCT patent applications, ~ 20 publications in high impact journals like JCR etc., 6 book chapters & ~ 60 presentations to his credit. He also has received 20 research awards including prestigious industry awards from Glenmark (Best innovation team – won twice), Merck (Innovation cup), Novartis (International bio-camp) & Swiss Society of Pharmaceutical Sciences (Best publication).
-
 Dr. Suneet ShuklaSenior Pharmacologist at FDA Washington D.C. Metro Area
Dr. Suneet ShuklaSenior Pharmacologist at FDA Washington D.C. Metro AreaDr Suneet Shukla is a pharmacist with Ph.D. in Molecular Pharmacology from Jawaharlal Nehru University, New Delhi, India. He completed his postdoctoral training at National Cancer Institute, NIH. He then joined FDA and has worked at the Office of Pharmaceutical Quality and the Office of Generic drugs where his responsibilities included the assessment of INDs, NDAs, ANDAs. Currently, he is working as Senior Pharmacologist at the Office of Clinical Pharmacology. DrShukla possesses years of highly specialized research and regulatory experience in PK/PD data analysis, in vitro drug release and dissolution analysis, bioequivalence determination, IVIVC model development and validation analysis for SUPAC changes or biowaiver applications, quantitative modeling and simulation analysis for small and large molecules from early to late drug development phase, drug-drug or drug-excipient interactions using transporter mediated IVIVE-PBPK model to understand drug absorption and oral bioavailability, DDIs (induction and competitive inhibition for liver, intestine, kidney) and pharmacogenomics in drug interactions.
DrShukla is on the Editorial Board of the Journal of Anticancer Research updates, serves as a peer reviewer for several journals, has published several highly cited research and review articles and has received patents and scientific awards from FDA and NIH. He has served on panels evaluating research grants submitted to NIH and FDA, mentored several undergraduate, graduate and post-doctoral trainees and currently serves as the lead instructor of the medical pharmacology course at the Foundation for Advanced Education in the Sciences (FAES) Graduate School, NIH.
The mission of CRS-IC is to promote education, create awareness, and to encourage scientific research towards the creation of intellectual wealth in the area of drug delivery systems in India. In an attempt to fulfill our mission, we have planned A National Level Workshop on “Manufacturing, Characterization, and Applications of Monoclonal Antibodies” on Saturday, 10th October 2020.
The recent years have seen phenomenal growth in the field of research and commercialization of monoclonal antibody-based therapeutics. With the right confluence of Pharmacy and Biotechnology, this field is set to progress by leaps and bounds. The workshop is hence designed to provide insight into the development, manufacturing, and applications of monoclonal antibody-based formulations.
Please find attached the flyer of the workshop and the link for registration.
E-certificates shall be provided to the participants upon successful completion of the workshop.
Click here to provide us with your valuable feedback post-event.
Abstracts and Speaker Profiles
Title of the talk: Development of human monoclonal antibodies
 |
Brief profile: Dr. K. Rajeshwari, Founder and Managing Director of Bioklone Biotech Private Limited, Chennai, India is a scientist turned entrepreneur. She obtained her Ph.D from Indian Institute of Science (IISc), Bangalore, India. She was a Post-Doctoral Fellow at Thomas Jefferson University, Philadelphia, USA and a Visiting Fellow at Tata Institute of Fundamental Research (TIFR), Mumbai, India before inception of Bioklone in 2006. Rajeshwari has been a resource person for several Entrepreneurship development programmes and has been a mentor for aspiring and start-up entrepreneurs. In 2015, Entrepreneurship Development Institute of India (EDII), Gandhi Nagar, Gujarat, India under the Aegis of Department ofScience & Technology published Dr. Rajeshwari’s case study in the science and technology space of a book entitled “The Innovators-Stories of Hi-tech Entrepreneurs”. |
Abstract of the talk
Ever since the development of hybridoma technology in 1975 by Kohler and Milstein, the use of monoclonal antibodies (mAbs) has become one of the major breakthroughs in medicine.Following the approval of the first therapeutic antibody in 1986 by the FDA; the production and uses of antibody therapeutics have dramatically changed. The emergence and evolution of antibody platforms designed to produce mouse chimeric, humanized and finally human antibodies was a result of the early recognition of the potential of mAbs as targeted therapeutics.
A mAb is derived from a single clone of cells and recognizes a unique antigenic determinant.
Murine mAbs are obtained from murine hybridomas produced by fusion of B lymphocytes from immunized mice or rats with murine myeloma cells. MAbs are routinely used in biochemistry, molecular and cellular biology, and medical research. One of the most beneficial applications of mAbs is their use as therapeutic drugs for the treatment of human diseases, such as cancer, asthma, arthritis, psoriasis, Crohn’s disease, transplant rejection, migraine headaches and infectious diseases.
The first successful human mAb (hmAb) with predefined specificity was developed in 1980;
through the fusion of human spleen cells from patients with human myelomas. Since then,
several methods have been established to generate hmAbs. These include (1) immortalization of antigen-specific human B cells; (2) acquisition of antigen-specific human B cells via phage display technology; (3) the production of hmAbs from transgenic mice; and (4) single human B cell cloning techniques to directly clone and express immunoglobulin (Ig) genes in vitro from antigen-specific B cells.
One of the major challenges in the development of hmAbs is the generation of human hybridoma cell lines of acceptable stability. It is also difficult in most cases to obtain antigen-primed human B lymphocytes suitable for fusions. To circumvent this, alternative strategies have been devised for the production of hmAbs, including (1) Fusion of human B lymphocytes with a murine myeloma or hybrid human-murine myeloma cell line; (2) Fusion of human B lymphocytes with a human lymphoblastoid cell line; (3) Transformation of human B lymphocytes with Epstein-Barr virus (EBV); and (4) Fusion of an EBV-transformed human B lymphocyte line with a mouse myeloma cell line.
The mAb market is growing at an increasing pace. Important advances in antibody engineering along with a greater understanding of the immunomodulatory properties of antibodies, have paved the way for the next generation of new and improved antibody-based drugs for the treatment of human diseases.
Title of the talk: Basics of Monoclonal Antibodies Drug Development
 |
Brief profile: DrSuneetShukla is a pharmacist with Ph.D. in Molecular Pharmacology from Jawaharlal Nehru University, New Delhi, India. He completed his postdoctoral training at National Cancer Institute, NIH. He then joined FDA and has worked at the Office of Pharmaceutical Quality and the Office of Generic drugs where his responsibilities included the assessment of INDs, NDAs, ANDAs. Currently, he is working as Senior Pharmacologist at the Office of Clinical Pharmacology. DrShukla possesses years of highly specialized research and regulatory experience in PK/PD data analysis, in vitro drug release and dissolution analysis, bioequivalence determination, IVIVC model development and validation analysis for SUPAC changes or biowaiver applications, quantitative modeling and simulation analysis for small and large molecules from early to late drug development phase, drug-drug or drug-excipient interactions using transporter mediated IVIVE-PBPK model to understand drug absorption and oral bioavailability, DDIs (induction and competitive inhibition for liver, intestine, kidney) and pharmacogenomics in drug interactions.
DrShukla is on the Editorial Board of the Journal of Anticancer Research updates, serves as a peer reviewer for several journals, has published several highly cited research and review articles and has received patents and scientific awards from FDA and NIH. He has served on panels evaluating research grants submitted to NIH and FDA, mentored several undergraduate, graduate and post-doctoral trainees and currently serves as the lead instructor of the medical pharmacology course at the Foundation for Advanced Education in the Sciences (FAES) Graduate School, NIH. |
Abstract of the Talk: Biological products are large, highly complex molecules derived from living cells or organisms and are the fastest growing class of therapeutic products in the United States. There are many types of biological products approved, including therapeutic proteins, monoclonal antibodies, and vaccines. The talk will focus on the characterization of monoclonal antibodies as drugs and important aspects of preclinical and clinical development of antibody drug development. Biosimilars, a class of biologic drugs that are highly similar to the reference product and can be approved under an abbreviated 351(k) biosimilar development pathway will also be discussed. Further, the concept of not independently establishing the safety and effectiveness of the biosimilar product and demonstration of no clinically meaningful differences between the biosmilar product and the reference product as it applies to reducing the residual uncertainty between two products will be discussed in this session.
Title of the talk: Role of antibodies based therapeutics in the modern healthcare system
 |
Brief profile: Dr. SachinDubey is presently working with Ichnos Sciences, Switzerland as Deputy Director & Head of the Formulation, Analytical & Drug Product Development. His current responsibilities include designing & executing product development & characterization strategies for both early & late-stage products for Ichnos Biologics. Sachin earned his Ph.D. from the University of Geneva, Switzerland & has previously worked with Glenmark Pharmaceuticals, Switzerland &NovozymesBiopharma, Denmark. He has ~ 12 years of experience in biopharmaceutical formulation & combinational product development. Twelve products developed by him & his team are currently in different clinical trials, he has overseen the production of >75 drug product/diluent/diluent for clinical trial phases 1-3. He has extensive experience with regulatory submissions in the USA, EU & India and have 9 PCT patent applications, ~ 20 publications in high impact journals like JCR etc., 6 book chapters & ~ 60 presentations to his credit. He also has received 20 research awards including prestigious industry awards from Glenmark (Best innovation team – won twice), Merck (Innovation cup), Novartis (International bio-camp) & Swiss Society of Pharmaceutical Sciences (Best publication). |
Abstract of the Talk: Monoclonal antibodies have made a significant impact on the management of several indications; many of them are now the first-line treatment for different oncology and autoimmune conditions. By virtue of being large, they are capable of not only binding to the target but also possess additional secondary functions – longer half-life by virtue of FcRn binding and ADCC/CDC being the most important. The roles of these additional functionalities are critical for their use in certain indications like oncology. Examples highlighting the impact of monoclonal antibody in the management of oncology/autoimmune conditions will be discussed. The development of antibody-drug conjugates (ADC’s) is another application of antibodies that will be discussed. Recent developments like bispecific and multi-specific antibodies and their promising applications will be highlighted. Producing monoclonal antibodies is a lengthy process and are often costly, however, the acceptance of biosimilars by regulatory agencies worldwide is expected to foster efforts in cost reduction and reach to the patient. Finally, the antibodies also find applications outside therapeutics ex. in diagnostics, purification etc. These additional applications will be summarized
Oral Delivery of Biologics
Local Time
- Timezone: America/New_York
- Date: Aug 29 2020
Speaker
-
 Dr. Yogeshwar BacchavFounder Director, Adex Pharma & Co-opted member of Executive Committee, CRSIC
Dr. Yogeshwar BacchavFounder Director, Adex Pharma & Co-opted member of Executive Committee, CRSICDr. Yogeshwar Bachhav is a Pharmacist by training. After completing PhD in Pharmaceutics from the Institute of Chemical Technology, Mumbai (Formerly UDCT), he worked in Europe (DACH region) for around 15 years with different start up and mid-size biotech and pharmaceutical companies.
During this tenure, he has successfully managed pharmaceutical development of several projects from POC to phase 1, 2 and 3 clinical trials. He has Strong network of Experts and CDMOs in EU, USA and Asia. In addition, he has 16 international publications, 8 patents and 30 conference proceedings to his credit. Recently he has edited a book for Wiley-VCH (Germany) on “Innovative Dosage Form, Design and Development at Early Stage”. Dr. Bachhav has been invited as a speaker for many international symposia around the globe (India, Europe and US).
He is a full time consultant with a German Biotech company -AiCuris Anti-infective Cures since 2014. There he is responsible to deal with pharmaceutical development of new drugs in anti-infectives segment.
In addition, Yogesh has founded a Pharmaceutical R&D consultancy firm – Adex Pharma in 2016. The prime objective of Adex is to resolve complex issues in the Pharmaceutical development of new drugs (INDs) and life cycle management of approved drugs (NDAs/ANDAs). He also supports few clients in India to resolve the CMC issues.
Dr. Bacchav is a member of the executive committee of Controlled Release Society-Indian Chapter.
On 29th Aug 2020, CRSIC conducted 2nd National level webinar titled “Oral Delivery of Biologics”. Dr. Yogeshwar Bacchav, Founder Director, Adex Pharma & Co-opted member of Executive Committee, CRSIC was an invited resource person for this technical talk. Oral delivery of biologics like proteins, peptides, and hormones especially insulin has always been a topic of great interest and extensive research. During his talk, Dr. Yogeshwar first presented the challenges in the oral delivery of biologics and then explained formulation strategies investigated so far, to overcome these challenges. His lucid and informative talk acquainted the participants with the latest nanotechnology based carriers and the advanced medical devices that are being explored for the effective oral delivery of the biologics.
Vaccine Manufacturing: Opportunities and Challenges
Local Time
- Timezone: America/New_York
- Date: May 02 2020
Speaker
-
 Dr. S. S PisalDirector- R&D & Manufacturing, Serum Institute of India Pvt Ltd., Pune
Dr. S. S PisalDirector- R&D & Manufacturing, Serum Institute of India Pvt Ltd., PuneDr. Pisal graduated from Shivaji University in 1991 and completed his Masters (1993) and Ph D (2002) from Pune University. He has guided 30 Masters and 5 Ph D students from IIT Madras, Symbiosis International University and several other Indian Universities like Savitribai Phule Pune University, Bharati Vidyapeeth Deemed University, Tilak Maharashtra Vidyapeeeth. His research group has won five national awards for the best research from AICTE. He has successfully completed several R&D projects for AICTE and Pharma as well as Biotech industry (Ventri Biologicals, Lupin Ltd, Aristo). He was appointed UGC Professor for Innovative Programme in Pharmaceutical Biotechnology at Poona College of Pharmacy, Pune.
Dr. Pisal has been associated with Serum Institute of India Pvt. Ltd (SIPL) since 2007 and currently working as a Director of R&D and Manufacturing. He also leads Intellectual Property Cel at SIPL. His groups at Serum have collaborations for vaccine/mAb therapeutics development with MIT Boston, Oxford University UK, PATH US, NIBSC UK, MBL US, Kansas University US. At SIPL, his groups have contributed to development of Anticancer formulations for US market, Rotavirus vaccine (world’s first heat stable vaccine), Rabies vaccine, IND monoclonals like Rabies human monoclonal (first time in the world), Dengue monoclonal antibody, Pentavalent meningococcal conjugate vaccine and Injectable polio vaccine.
Dr. Pisal currently holds 26 Indian/International patents/applications encompassing formulation development, lyophilization, upstream/downstream process development for vaccines and mAbs and vaccine delivery. He is the author of 40 peer-reviewed journal articles. Dr Pisal, on behalf of SIIPL, received the globally coveted “United States Patent Office’s Patents for Humanity Award- 2018- Medicine Category” in Nov 2018. SIIPL IP Cell received coveted “IP Excellence Award (Life sciences) at Questel IP Executive Summit in Aug 2019. Dr. Pisal has been an invited expert for 1. Bill and Melinda Gates foundation for deciding funding strategy for vaccine projects, 2. Indian Council for Medical Research for filing IP on polio diagnostics and, 3. WHO / MSF for IP inputs on new vaccines for developing countries.
Amidst the lockdown due to COVID Pandemic, Indian Chapter of CRS organized its very first National webinar titled “Vaccine Manufacturing: Opportunities and Challenges” on 2nd May 2020. Dr. S. S. Pisal was an invited speaker for this webinar. Dr. Pisal is currently working as a Director -R&D & Manufacturing at Serum Institute of India, Pvt. Ltd., Pune. Serum institute is one of the largest vaccines manufacturers of the globe.
With the long standing experience in the field of vaccine formulation development, upstream/downstream processing of vaccines, Dr. Pisal delivered an insightful and enlightening talk on the theme of the webinar. He explained the intricate details of vaccine development focusing on lyophilization process, one of the most crucial steps during vaccine manufacturing. The national webinar was well received by more than 100 participants.



